2-Methylheptane
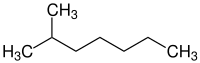
2-Methylheptane is a colourless liquid, chemical compound which is part of the branched alkane family and is isomeric to octane Where a methyl group has been added to the second carbon in heptane. Its structural formula is (CH3)2CH(CH2)4CH3.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylheptane[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
1696862 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.863 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1262 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C8H18 |
| Molar mass | 114.232 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | Odourless |
| Density | 698 mg mL−1 |
| Melting point | −112 to −108 °C; −170 to −163 °F; 161 to 165 K |
| Boiling point | 116.8 to 118.4 °C; 242.2 to 245.0 °F; 389.9 to 391.5 K |
| Vapor pressure | 5.3 kPa (at 37.7 °C) |
Henry's law constant (kH) |
2.7 nmol Pa−1 kg−1 |
Refractive index (nD) |
1.395–1.396 |
| Thermochemistry | |
Heat capacity (C) |
252.00 J K−1 mol−1 |
Std molar entropy (S⦵298) |
356.39 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−256.5–−253.9 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−5466.7–−5464.3 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
Pictograms |
    |
Signal word |
Danger |
Hazard statements |
H225, H304, H315, H336, H410 |
Precautionary statements |
P210, P261, P273, P301+P310, P331 |
| NFPA 704 (fire diamond) | 
0
3
0 |
| Flash point | 4.4 °C (39.9 °F; 277.5 K) |
| Explosive limits | 0.98–?% |
| Related compounds | |
Related alkanes |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
If the standard definition of the prefix "iso-" is strictly used then 2-Methylheptane can be called "Isooctane", however this name is usually used for another much more important isomer of octane 2,2,4-Trimethylpentane[3]
References
- "2-METHYLHEPTANE - Compound Summary". PubChem Compound. National Center for Biotechnology Information. 26 March 2005. Retrieved 6 March 2012.
- PubChem. "2-Methylheptane". pubchem.ncbi.nlm.nih.gov. Retrieved 2024-02-18.
- Clayden, Jonathan (2005). Organic chemistry (Reprinted (with corrections). ed.). Oxford [u.a.]: Oxford Univ. Press. pp. 315. ISBN 978-0-19-850346-0.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.