α-Galactosidase
α-Galactosidase ( EC 3.2.1.22, α-GAL, α-GAL A; systematic name α-D-galactoside galactohydrolase) is a glycoside hydrolase enzyme that catalyses the following reaction:[1]
- Hydrolysis of terminal, non-reducing α-D-galactose residues in α-D-galactosides, including galactose oligosaccharides, galactomannans and galactolipids
| α-galactosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 α-Galactosidase tetramer, Mortierella vinacea | |||||||||
| Identifiers | |||||||||
| EC no. | 3.2.1.22 | ||||||||
| CAS no. | 9025-35-8 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
It catalyzes many catabolic processes, including cleavage of glycoproteins, glycolipids, and polysaccharides.
The enzyme is encoded by the GLA gene.[2]
Function
This enzyme is a homodimeric glycoprotein that hydrolyses the terminal α-galactosyl moieties from glycolipids and glycoproteins. It predominantly hydrolyzes ceramide trihexoside, and it can catalyze the hydrolysis of melibiose into galactose and glucose.
Reaction mechanism
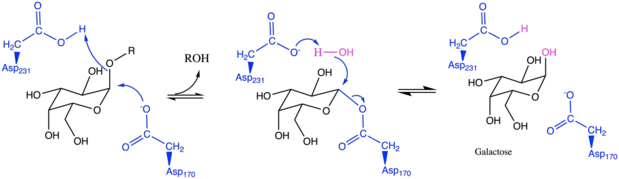
A double displacement reaction mechanism of α-GAL's catalytic action.The ligand (black) when bound in the active site of the enzyme (blue). The two key amino acid residues in the active site are Asp-170 and Asp-231. First, Asp-170 performs a nucleophilic attack on the glycosidic bond to release the terminal α-galactose molecule from the ligand. Then, Asp-231 serves as an acid to remove a proton from water, making it more nucleophilic to attack the galactose-Asp complex and release α-galactose from the active site.[3][4][5]
See also
- β-galactosidase
- Migalastat, a drug targeting α-galactosidase
- Classification of α-galactosidases (according to CAZy)
References
- Scriver CR, Sly WS, Childs B, ABeaudet AL, Valle D, Kinzler KW, et al. (15 December 2000). The Metabolic & Molecular Basis of Inherited Disease (8th ed.). McGraw-Hill. ISBN 978-0-07-913035-8.
- Calhoun DH, Bishop DF, Bernstein HS, Quinn M, Hantzopoulos P, Desnick RJ (November 1985). "Fabry disease: isolation of a cDNA clone encoding human α-galactosidase A". Proceedings of the National Academy of Sciences of the United States of America. 82 (21): 7364–8. Bibcode:1985PNAS...82.7364C. doi:10.1073/pnas.82.21.7364. PMC 391345. PMID 2997789.
- Koshland DE (1953). "Stereochemistry and the Mechanism of Enzymatic Reactions". Biological Reviews. 28 (4): 416–436. doi:10.1111/j.1469-185x.1953.tb01386.x. S2CID 86709302.
- Brumer H, Sims PF, Sinnott ML (April 1999). "Lignocellulose degradation by Phanerochaete chrysosporium: purification and characterization of the main α-galactosidase". The Biochemical Journal. 339 (1): 43–53. doi:10.1042/bj3390043. PMC 1220126. PMID 10085226.
- Vocadlo DJ, Davies GJ (October 2008). "Mechanistic insights into glycosidase chemistry". Current Opinion in Chemical Biology. 12 (5): 539–55. doi:10.1016/j.cbpa.2008.05.010. PMID 18558099.
External links
- alpha-Galactosidase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human GLA genome location and GLA gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.