3-PGA ($\ce{C_6H_7O_7P}$) is converted to G3P ($\ce{C_6H_7O_6P}$) before it is able to be made into glucose, with an expansion of 6 ATP and 6 NADPH. My question is why this process is needed and why the loss of 1 oxygen atom decides whether a sugar molecule can be synthesized into glucose. My take on this matter is that if they incorporate 3-PGA directly into glucose, they can save time and also energy in the form of ATP and NADPH.
-
1Obviously not a complete answer, but I wonder if it's partially because NADP+ is needed to absorb the electrons and protons at the end of the ETC at PS-I to avoid a surplus of NADPH and H+, resulting in a dangerously acidic environment. Good question, btw. – rotaredom Jan 30 '18 at 19:10
-
@rotaredom — Please observe the text that appears in the comment box: "Use comments to ask for more information or suggest improvements Avoid answering questions in comments." Comments are not for answers, complete or incomplete. – David Jan 30 '18 at 21:50
-
- Please refer to chemical compounds by their names as well as their abbreviations for clarity and indexing. 2. This is a question of chemistry which is covered in any biochemistry text. Your naive remark about "saving time and energy" and your emphasis on irrelevant chemical compositions suggests that you should consult such a text before posting. I suggest Berg et al. online Chapter 20. This wouldn't be a homework question by any chance?
– David Jan 30 '18 at 21:59 -
Although I liked answering this question, it did not take me more than 5 minutes to gather all the required stuff for this answer. From next time, @user35897, please show some research effort before asking a question. As of now, I am voting to close this question. – another 'Homo sapien' Feb 01 '18 at 18:07
1 Answers
Nice question! To see what trouble that extra oxygen can cause, lets get to the core of the reaction i.e. Calvin-Benson cycle; one step at a time. To start with, have a look at the detailed Calvin-Benson cycle (following image taken from here):
As becomes quite clear from this image, the actual use of Glyceraldehyde-3-phosphate is at the step of formation of Fructose-1,6-bisphosphate. Yet, we don't know why we cannot use 3-Phosphoglycerate instead at this step. For that, lets go a step further and see how the concerned enzyme, fructose-1,6-bisphosphate aldolase/phosphatase (aka aldolase), works.
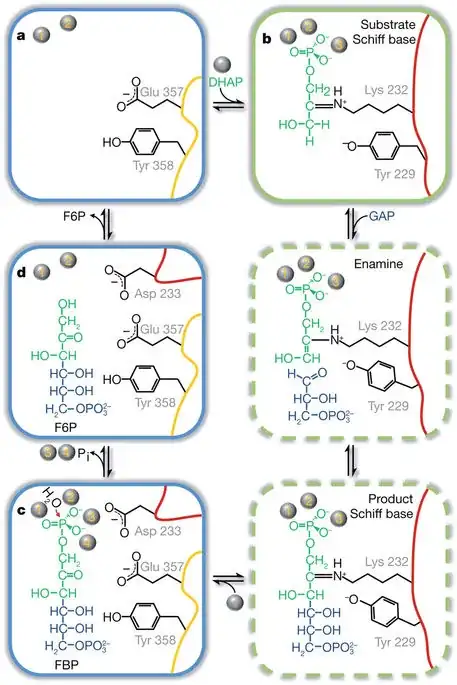
Since the full article is behind a paywall, lets get a sneak peak into it. See this image1:
Pay attention to the steps where G3P enters i.e. steps between b and c. Even without entering into details of the mechanism, we get two factors which can prevent 3PGA from being utilized in this reaction:
Steric Hindrance: replace the terminal -H with -OH of G3P in the first image in your mind. You can clearly see that this would cause some steric hindrance and prevent formation of covalent bond between DHAP and G3P.
Formation of Geminal Diol: again, replace the terminal -H with -OH, but this time in the second image, in your mind. You get geminal diol in the resultant molecule, which is famous for its reactivity and instability. Thus, by adding 3-PGA instead of G3P in the reaction, we might end up with unstable molecules that cleave themselves up. I hope this is what you were looking for.
- 14,121
- 5
- 60
- 92
-
Hi sorry I missed your answer. So just to clarify, the reasons are 1)NADP+ is needed to absorb the electrons and protons at the end of the photosystem 1 electron transport chain, or else it would result in an acidic environment in the chloroplast. 2)It will prevent covalent bonding between the DHAP and G3P, causing steric hindrance. 3)An unstable molecule (Geminal Diol) would be formed, which will cleave itself much like the intermediate 6-carbon molecule which was formed after carbon fixation. – user35897 Mar 15 '18 at 10:07
-
-
The first point seems to come from @xusr's answer, so I cant be so sure about it. The other two points seem just fine though (sorry for being late, I really cant get time to access this site) – another 'Homo sapien' Mar 20 '18 at 20:22
-
Thanks for the help! Much appreciated. I tried researching on this myself actually but I couldn't find anything useful – user35897 Mar 21 '18 at 12:45
-