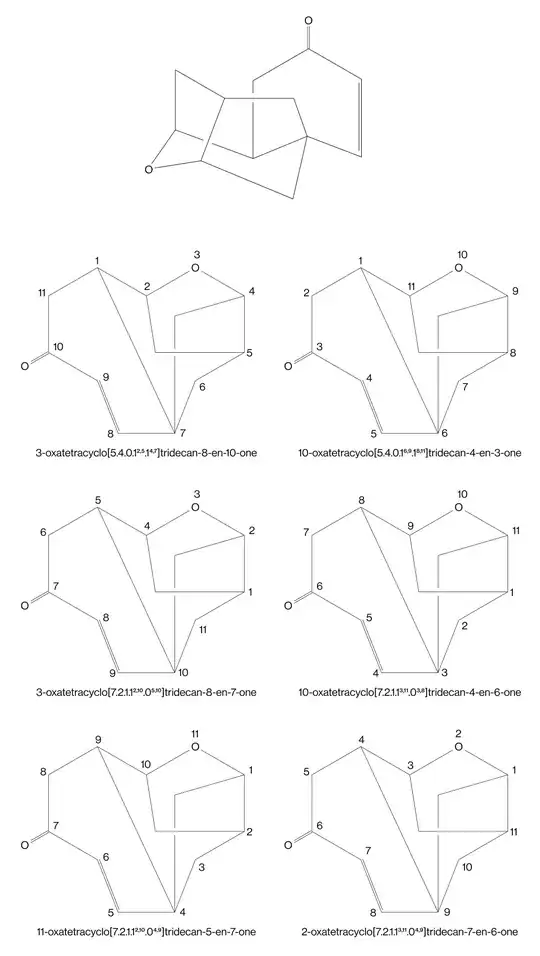
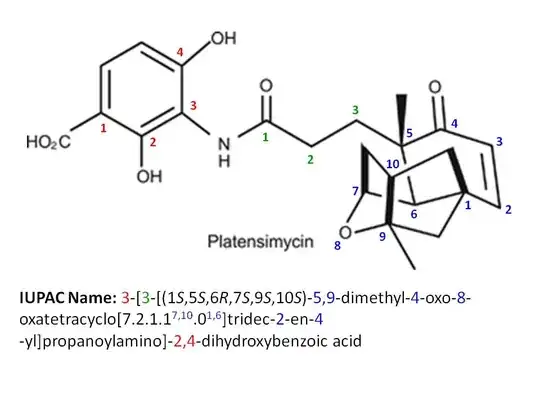
The pubchem website has given Platensimycin IUPAC name, $3$-$[3$-$[(1S,5S,6R,7S,9S,10S)$-$5,9$-dimethyl-$4$-oxo-$8$-oxatetracyclo$[7.2.1.1^{7,10}.0^{1,6}]$tridec-$2$-en-$5$-yl]propanoylamino]-$2,4$-dihydroxybenzoic acid (see attached picture):
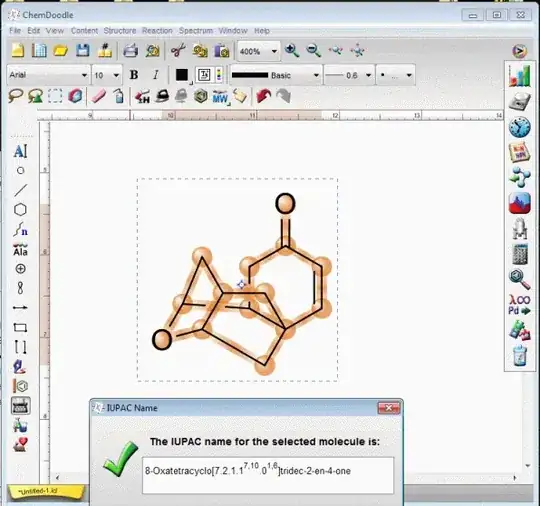
Accordingly, since https://pubchem.ncbi.nlm.nih.gov is usually updating data according to the changes, I believe the name of the cyclic system (without 5,9-dimethyl groups) OP is asking is same as one in the comment by @KarstenTheis (including the correct stereochemistry): $(1S,5S,6R,7S,9S,10S)$-$4$-oxo-$8$-oxatetracyclo$[7.2.1.1^{7,10}.0^{1,6}]$tridec-$2$-ene (see the blue numbering in the picture).
Late correction: After exchanging few comments with Loong, I realized that the given system should be treated as a chemical compound. Accordingly, the name should have been: $(1S,5S,6R,7S,9S,10S)$-$4$-oxo-$8$-oxatetracyclo$[7.2.1.1^{7,10}.0^{1,6}]$tridec-$2$-en-$4$-one (as suggested by Loong).