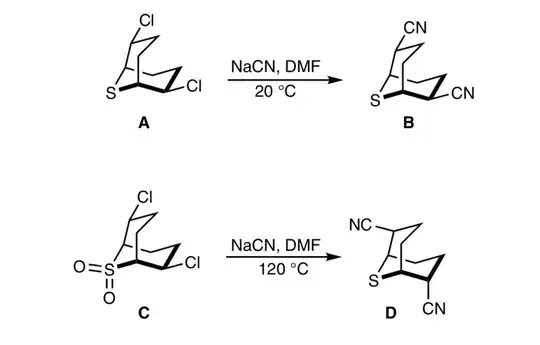
In this question there is no inversion in A to B, but there is one in C to D. I feel like this must be due to the lone pairs on the $\ce{S}$ (in A) being able to open the ring, whereas this isn't possible in C to D so this can only occur via an $\mathrm{S_N2}$ reaction, but I just can't seem to make the mechanisms work for either.
Asked
Active
Viewed 59 times
2
-
2Remember S is a significantly larger atom than O, and episulfonium ions are well known – Waylander May 20 '19 at 12:25
-
1Instead of opening (i.e. breaking) rings, think about forming rings, maybe – orthocresol May 20 '19 at 12:30
-
1So is the sulphur lone pair able to attack via SN2 to form an episulfonium ring, which is then attacked by SN2 with the CN ions, meaning there is no inversion? – J. Deans May 20 '19 at 16:45
-
That is exactly so – Waylander May 20 '19 at 17:48
1 Answers
3
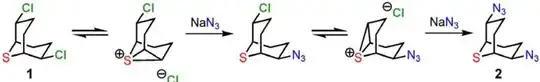
Because of the absence of a lone pair, C undergoes usual $\mathrm{S_N2}$ transformation under the conditions. However, the availability of a lone pair in A undergoes the nucleophilic substitution of cyanide ion via neighboring-group participation (see above comments by Waylander and J. Deans) by the sulfur atom under the conditions (Ref.1). A very reliable mechanism is given in Ref.1 for the substitution of azide ion (work the same way for cyanide ion):
Reference:
- D. D. Díaz, A. Converso, K. B. Sharpless, M. G. Finn, “2,6-Dichloro-9-thiabicyclo[3.3.1]nonane: Multigram Display of Azide and Cyanide Components on a Versatile Scaffold,” Molecules 2006, 11(4), 212–218 (doi: 10.3390/11040212).
Mathew Mahindaratne
- 39,943
- 27
- 55
- 107
-
Could you check out the duplicate of the question, and maybe post another (essentially the same) answer there? – Martin - マーチン May 20 '19 at 18:01
-
-
I think it's fine. You can add a disclaimer that you have posted the answer on another question before. Or if you feel more comfortable, you can post your answer there and then flag this one for deletion (you can't delete yourself as it is accepted). I think it would be beneficial to have the duplicate link point to a question that is actually answered. We could also have a meta discussion about it, of you want to make absolutely sure. – Martin - マーチン May 20 '19 at 18:38
-
@Martin - マーチン: Thank you Martin for your lengthy explanation. Let me think about it. Previous question is asking to show the stereochemistry to prove the out come of the products. I think this may do that. :-) – Mathew Mahindaratne May 20 '19 at 19:22
-
Thank you very much for your answer. Very sorry I was unaware the question had been posted before – J. Deans May 20 '19 at 21:55
-
@Martin - マーチン: I put a different answer for duplicate but with same figure. Thanks. – Mathew Mahindaratne May 21 '19 at 15:53
-
@MathewMahindaratne I've seen and cited on it. Thanks a lot for doing this. – Martin - マーチン May 21 '19 at 17:43