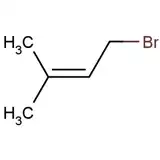
What is the product of the reaction of 2-methylbuta-1,3-diene (isoprene) with HBr?
What I understand is that carbon (2) will get a positive charge after protonation.
But after this, which should be the major product?
- 3-bromo-3-methylbut-1-ene – because in this case the carbocation would be stable because of being tertiary, but the double bond would have less number of hyperconjugated structures and will be less stable
- 1-bromo-3-methylbut-2-ene – as the carbocation would be less stable because it would be primary but the double bond would be much more stable because of 8 $\alpha$ hydrogen available