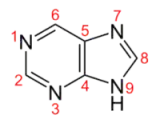
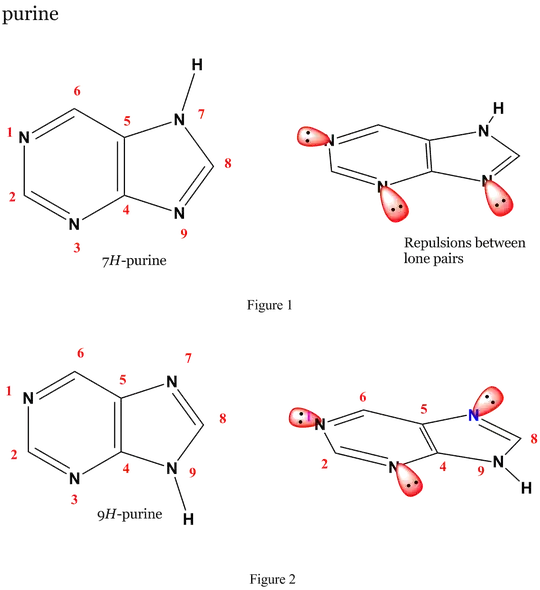
I tried to explain the equilibrium by comparing the basicity of different nitrogen atoms in a purine anion, but I can’t see any obvious difference. Seems like that the 9-N will be the most basic because it can have two adjacent imine structure while keeping the structure of pyrimidine untouched. Computational results will be appreciated.
P.S. I don’t think the question is a duplicate of the “purine nitrogen basicity” one because that one compares the basicity of nitrogens in a neutral purine molecule whereas my question compares the basicity in a purine anion.