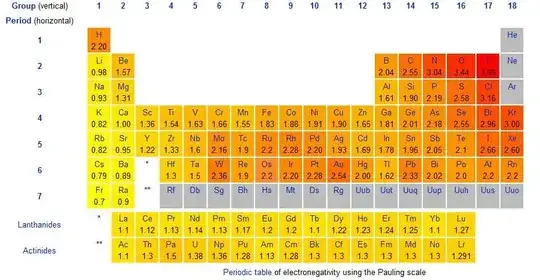
I recently learnt that electronegativity generally decreases as I move down a group and from right to left within a period. However, according to the table below, Pb has an electronegativity of $2.33$, which is higher than $1.96$ of Sn. There are also a lot other elements, such as Au, Hg, and W, that does not follow the general rule of electronegativity.
Can someone offer an explanation of why some elements have a higher electronegativity than the elements directly above them?