Phys.org's Accelerating chemical reactions without direct contact with a catalyst discusses the new Science Advances paper Noncontact catalysis: Initiation of selective ethylbenzene oxidation by Au cluster-facilitated cyclooctene epoxidation. I expected to read about something really unusual, long range forces, virtual entangled photons...
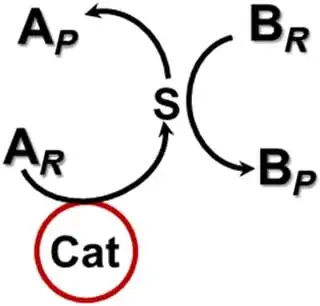
But Figure 1 of the paper (shown below) helps to explain that the catalyst produces an intermediary species and it's this that initiates or catalyzes the desired effect.
Question: Is it possible to make clear to a non-chemist how new the published noncontact catalytic system reaction really is? What is it that makes this unique or novel in 2020? Did nobody think of this until now, or have people been trying for a long time but this is the first success?
Fig. 1 A noncontact catalytic system.
A catalyst (Cat) catalyzes reaction A (AR ➔ S ➔AP) in which the intermediate S is effective in either initiating or catalyzing reaction B (BR ➔ BP), although reaction B is not catalyzed by the catalyst.