I heard that spontaneous reaction happens if $\Delta G=\Delta H-T\Delta S$ is negative.
For combustion of methane, according to Chemguide:
- $\Delta H=\pu{-891.1 kJ K^{-1} mol^{-1}}$
- $\Delta S=\pu{-0.2422 kJ K^{-1} mol^{-1}}$
Thus, I would deduce that at normal temperature, $\Delta G<0$, combustion of methane is spontaneous, while at very high temperatures, it will stop.
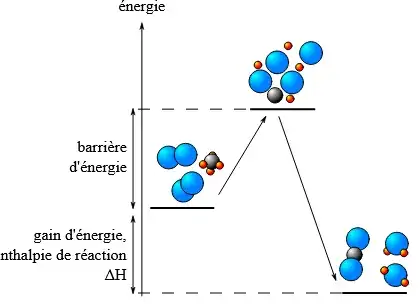
But, from another reference, I deduce the opposite conclusion: if we consider the diagram below (taken from https://fr.wikipedia.org/wiki/R%C3%A9action_chimique),
I would think that in order to be able to go to the intermediate state, one needs to provide energy, thus that it could not be spontaneous for this reason. One needs to provide energy, for example by heating, that is increasing temperature.
How is that possible? Any comment?