Since the overlap increases with directional properties of orbital,
$$\ce{p - p > s - s > s - p}$$
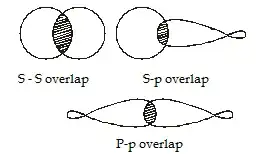
However it is also observed that the bond strength of
$$\ce{H-F > H-H > F-F}$$ $$\ce{\{s - p > s - s > p - p\}}$$
Why does this happen? I've done some research and found this which says that it may depend on the p orbital's alignment with inter-nuclear axis.
The reference doesn't seem to apply here, since all the above orbitals are aligned along the inter-nuclear axis.
What am I missing here?
Bond dissociation enthalpies:
$$\begin{array}\\ \ce{H-F} &&& \pu{568 kJ/mol} \\ \ce{H-H} &&& \pu{436 kJ/mol} \\ \ce{F-F} &&& \pu{157 kJ/mol} \\ \end{array} $$ Source