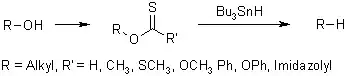I have been looking everywhere for hours now, and can't find anything on a simple lab reduction of an alcohol to an alkane that doesn't involve any "fancy" reagents such as tosylates, metal catalysts etc.
I would have expected that under acidic circumstances, the oxygen donates an electron to the proton from the acid, and a hydride from a general reducing agent such as LAH or NaBH4 then attaches itself to the carbon, pushing out a molecule of water, and alkane.
However, I have found no proof or mention of the existence of such a reaction. If this is is impossible, then why, and what would be the best general purpose way to reduce an OH to a H