Hydrogen peroxide has an "open book" structure:
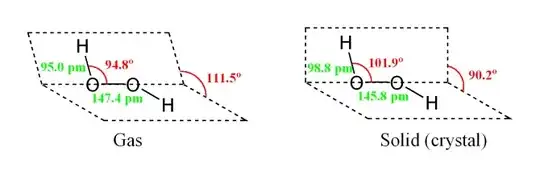
The dihedral angle in gaseous H$_2$O$_2$ is close to what we might expect in a tetrahedral arrangement. However, in solid H$_2$O$_2$ it's almost a right angle. My book says that this is due to "hydrogen bonding" but doesn't go into further detail.
Note that this question isn't a duplicate of Dihedral angle of gaseous and crystalline HOOH as the accepted answer is clearly incomplete, and the other answer uses a computer simulation that falls short of an intuitive explanation.