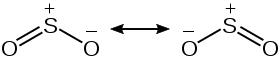
Triatomic molecules are either linear or bent. If we analyse the $\ce{SO2}$ molecule, it turns out that it is bent.
I know that $\pi$ bonds do not alter shape, but merely the bond lengths.
Now since one of the bonds between S and O is a p$\pi$-p$\pi$ bond and the other bond is a d$\pi$-p$\pi$ bond, the two $\pi$ bonds are not same, so the bond length should not be the same, but my book says the bond lengths are the same which is quite puzzling. I thought maybe its due to resonance but there's no way resonance takes place here. I read this question,but it don't answer many questions as:
Why d-orbitals of sulphur cannot be used ? And in answer by @Kenny Lau , what's specify reason for 'Sulfur sharing two electrons among itself and the other two oxygen atoms.'
So, that makes me think then, what could be the reason for same bond length in $\ce{SO2}$?
It is not clear as to why d-orbitals of sulphur cannot be used and in the answer to this question @Kenny Lau does not specify reason for 'Sulfur sharing two electrons among itself and the other two oxygen atoms.'