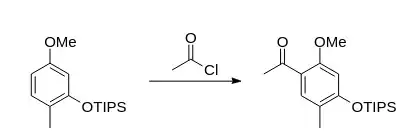
I am attempting to form a synthetic route to a tetrasubstituted benzene compound. In the scheme, there is a step that requires the formation of a carbon–carbon bond in the C-6 position of a methoxy group in a trisubstituted benzene ring (there is a silyl protected phenol in the 3 position and an alkyl group in the 4 position). Instead of the actual structure I may not disclose, below, I drew a structural prototype in the conversion intended:
I would like to complete the transformation via a Friedel–Crafts acylation because I have heard that anisoles, though not phenols, are suitable for performing Friedel–Crafts reactions. However I have also read that aluminum chloride can be used to demethylate anisole derivatives. I would like to know if I need to worry about demethylating the methoxy group and, more interestingly, if I do not, why?