What is the reason for the boiling point of benzene-1,4-diol being higher than that of benzene-1,3-diol?
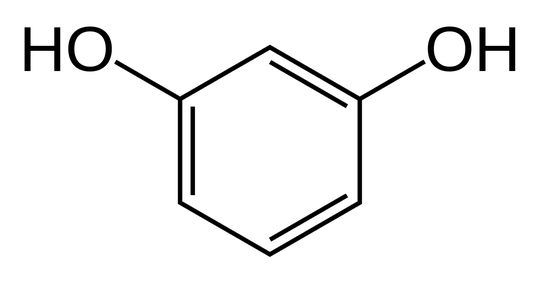
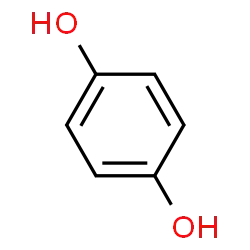
Both have hydrogen bonds. I think it is due to quinol's symmetry but can't point out exactly why this is the case.
What is the reason for the boiling point of benzene-1,4-diol being higher than that of benzene-1,3-diol?


Both have hydrogen bonds. I think it is due to quinol's symmetry but can't point out exactly why this is the case.
Benzene-1,4-diol has a boiling point of 287°C and Benzene-1,3-diol has a boiling point of 277°C and Benzene-1,2-diol has a boiling point of 245.5°C. This could be attributed to the ease of formation of 2 intermolecular hydrogen bonds with the 2 hydroxy groups of these molecules increasing when the hydroxy groups are placed apart, to minimise steric hindrance and repulsion. Benzene-1,4-diol could form intermolecular hydrogen bonds with more stability than Benzene-1,3-diol or Benzene-1,2-diol as these two will get destabilised by steric repulsion when large groups have to reach nearer to form hydrogen bonds.
Thus, the extent of intermolecular hydrogen bonding in : Benzene-1,4-diol > Benzene-1,3-diol > Benzene-1,2-diol
To overcome this attraction, more energy is needed. Hence the same is the order of their Boiling Points.