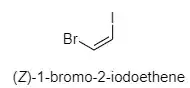
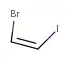
You have figured out the Z part of the nomenclature correctly. Now as to the question of why 1-bromo-2-iodo... and not 1-iodo-2-bromo..., we look at section P-14.4 (g) of the Nomenclature of Organic Chemistry. IUPAC Recommendations
and Preferred Names 2013, which states the following:
P-14.4 NUMBERING
When several structural features appear in cyclic and acyclic compounds, low locants are assigned to them in the
following decreasing order of seniority:
[...]
(g) locants for the substituent cited first as a prefix in the name;
According to this rule, the priority in numbering depends upon where each prefix is present within the name. You cannot have 2-bromo-1-iodo... or 2-iodo-1-bromo... as a possible name when the substituents are simple substituents.
Now, how do we decide which comes first? Is it the bromine or iodine group? For this we look into section P-14.5 which deals with alphanumeric order and states:
P-14.5 ALPHANUMERIC ORDER
[...]
P-14.5.1 Simple prefixes (i.e., those describing atoms and unsubstituted substituents) are arranged alphabetically;
multiplicative prefixes, if necessary, are then inserted and do not alter the alphabetical order already established.
Therefore, since bromo- comes first alphabetically, we place it first before iodine and by P-14.4 (g), the bromine gets priority.
Therefore, the PIN of the given compound becomes (Z)-1-bromo-2-iodoethene