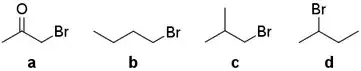
Compare the rates towards SN2 reaction:
I got the point that 2-bromobutane d is a secondary halide, so the steric hinderance is highest making it least reactive among the four. Both b and c are primary halides, but c has branched isobutyl group, so because of steric effect the order should be b > c > d.
However, the answer claims that bromoacetone a is the most reactive towards SN2 despite also being primary halide. What is the exact reason for this?
My friend says if there is carbonyl on β-position, then it is highly reactive towards SN2. Is this even accurate? If yes, then what is the reason?