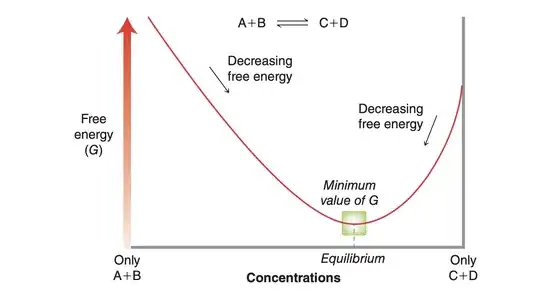
In the case of activation energy diagrams in terms of potential energy, I understand that when we're talking about two individual molecules, the potential energy is highest in the transition state since it is the most unstable, and then falls.
When this activation energy diagram is expressed in terms of gibbs free energy:
As is pointed out by this answer,here, they indicate that for the individual molecule interpretation, macro properties such as quantities such as temperature and pressure are ill-defined.
However, if we treat this as representing the reaction macroscopically, that can't be the case also since that would violate the second law (gibbs free energy rising). You would instead expect something like this diagram: