A chiral carbon atom is defined as
"a carbon atom with four different groups attached to it."
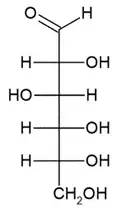
I am aware that the open-chain form of glucose has four chiral carbon atoms.
However, I do not undertstand why that is, since the alleged chiral atoms do not have four different substituents: For example, the 3rd carbon atom is bound to one hydrogen atom, one hydroxylgroup and two neighbouring carbon atoms.
@user55119compiled instructive answers; just two of them here, and here. – Buttonwood Feb 21 '23 at 13:45