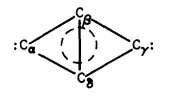
Ring strain and principles of aromaticity render such a molecule unstable.
Each double bond contributes 2 π electrons, and with four double bonds in one cycle in common, there is a total of 8. Compounds with (4n + 2) π electrons (where n is an integer number) are considered as Hueckel aromatic with an additional energetic stabilization e.g., in benzene compared to a hypothetical cyclo-1,3,6-cyclohexatriene. Compounds with 4n π electrons (e.g., 8) however are considered as anti aromatic – not only that they do not benefit the stabilization, they are extra unstable.
It does not say that there is not such a molecule (chemistry in stars / in mass spectroscopy allows for molecules and ions you can't store in bottles on the shelf), but – because of lack of overlap of atomic orbitals to establish bonds and thermodynamics (enthalpy of formation) – very unlikely.
Consider yourself lucky that the molecule set's bonds did not break constructing a cyclobuta-1,2,3,4-tetraene.