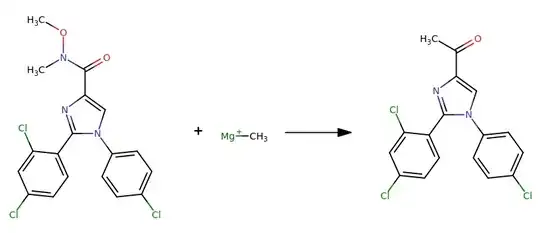
I am not able to identify the molecule C[Mg+]. I came across it a number of times in the USPTO-50k dataset, in reactions such as the one shown below:
CON(C)C(=O)c1cn(-c2ccc(Cl)cc2)c(-c2ccc(Cl)cc2Cl)n1.C[Mg+]>>CC(=O)c1cn(-c2ccc(Cl)cc2)c(-c2ccc(Cl)cc2Cl)n1
I searched in PubChem and MolView and couldn't identify the compound. The closest thing I found was magnesium carbide, with SMILES [CH3-].[CH3-].[Mg+2]. Is it the same compound?
Any ideas what C[Mg+] is or where I can look next? Also RDKit accidentally generated a similar molecule once, C[MgH]. Does this one exist too?