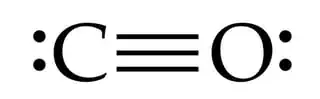I think you are taking a view of the bonding which is very ionic in nature. Consider for a moment the bonding in water. Water has a sp3 oxygen which can be regarded as being a distorted tetrahedral atoms. The water has two sigma bonds between the oxygen atom and the hydrogens and a total of two lone pairs on the oxygen.
What you have in carbon monoxide is a sp oxygen and a sp carbon, you can think of carbon monoxide as being similar to acetylene and dinitrogen.
Between the carbon and the oxygen in CO we have a sigma bond, we also have two pi bonds which are formed from the p orbitals on the carbon and oxygen atoms. These pi bonds are at 90 degrees to each other. It is important to understand that the electrons in a covalent bond are shared between the atoms rather than kept by one atom alone.
It is hard to know what you mean in the biro drawing, do you mean that the oxygen has two lone pairs which are off the axis of the C-O bond or do you mean something else. If this is what you mean then the oxygen would be a sp2 hydridized atom.
If we consider where the lone pair is on a carbon monoxide then it will help to look at S.Niibayashi, K.Mitsui, K.Matsubara, H.Nagashima, Organometallics, 2003, 22, 4885, DOI: 10.1021/om0340701. In this paper they report the strucutre of a metal complex where the lone pair of the oxygen bonds to a titanium.
The Ti-O-C angle is 162 degrees which is only 18 degrees away from a perfect 180 degrees. I think it is likely to be packing effects which have distorted the bond away from 180 degrees. Here is a picture of the complex.

The differece in electronegativity of the carbon and the oxygen cause the covalent bonds to be polarized such that the oxygen gets more electron density than the carbon. As a result the oxygen end of the carbon monoxide is more negative than the carbon end.
So if anything the oxygen is more negative than the carbon.