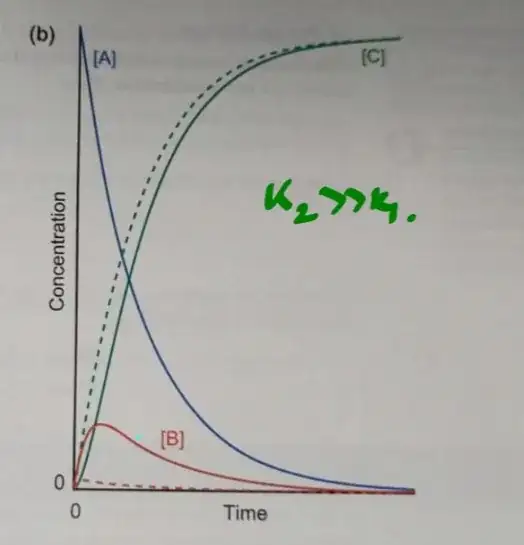
In graph given below is a sequential first order reaction
$$\ce{A ->[$k_1$] B ->[$k_2$] C}$$
For $k_2 \gg k_1$ the graph of concentration of $\ce{A}$, $\ce{B}$ and $\ce{C}$ is as follows.
Won't the graph of the concentrations of $\ce{B}$ and $\ce{C}$ be the same as if $\ce{B}$ is formed then only $\ce{C}$ will be formed simultaneously? Then why is $\ce{C}$'s graph different?