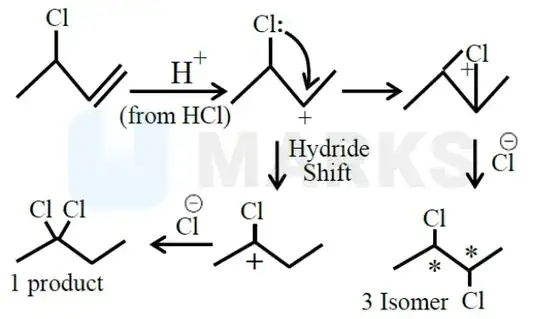
 Shouldn't the carbocation be formed away from the -I effect of the Cl atom for more stability. Backbonding is achieved in a later step after hydride shift.
Shouldn't the carbocation be formed away from the -I effect of the Cl atom for more stability. Backbonding is achieved in a later step after hydride shift.

Asked
Active
Viewed 44 times
1 Answers
0
That would result in a primary carbocation. Due to substituent groups stabilizing the carbocation's empty p-orbital in what's known as hyperconjugation, primary carbocations are less stable than secondary. Here are some heats of formation from the Active Thermochemical Tables (version 1.130):
t-Butylium (tertiary carbocation): 710.72 $\pm$ 0.98 kJ/mol
sec-Butylium (secondary carbocation): 769.0 $\pm$ 1.6 kJ/mol
n-Butylium (primary carbocation): 803.1 $\pm$ 1.2 kJ/mol
While tertiary carbocations are more stable than secondary ones, an electron-withdrawing group such as a chloro group destabilizes the carbocation.
Bernpout532
- 26
- 2
-
Right. So basically we can say that here we get more number of alpha H so more hyper conjugation hence, inspire of greater -I, carbocation will be formed there due to compensation by hyper conjugation. Thanks a lot. – Meet Shah Jan 13 '24 at 15:16
-
Hyperconjugation stabilizes the carbocation by electron donating groups (with p orbitals) adjacent to the empty p orbital of the positive formal charge carbon, not the destabilizing effect of alpha hydrogens. – Bernpout532 Jan 14 '24 at 06:05