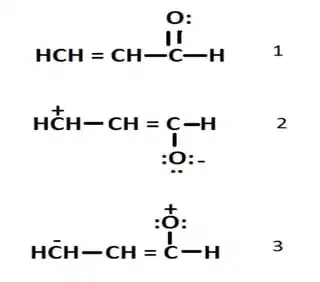
In a compound like acrolein (prop-2-enal or $\ce{CH2=CH-CHO}$, will the oxygen in the $\ce{-CHO}$ group always have a $\delta$- charge?
Acrolein does have three resonance structures as shown below, in which on one of them, oxygen has a $\delta$+ charge.
How stable is the third resonating structure? Is it viable to even consider it a resonating structure, since $\ce{-CHO}$, a decently strong minus I group has a $\delta$+ charge, and $\ce{-CH=CH2}$, a very weak minus I group has a $\delta$- charge?
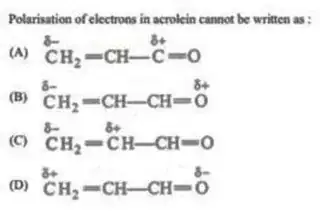
This doubt stemmed from the following question, about the resonant structures of acrolein (apologies for the low quality):
It's a multiple correct MCQ, and the answer given is $ABC$, but as per the resonating structures of acrolein, it should be $AC$, as B and D, both should be valid resonating structures of acrolein.