1) Why is only triphenyl phosphine used here. Why not some stronger
nucleophile like say trimethyl phosphine?
Trialkyl phosphines can particpate in the Wittig reaction just fine, but they create some other problems. Let's say you were going to create the phosphonium salt from ethyl iodide and trimethyl phosphine. It would form in high yield, but in the next step where you deprotonate it and form the ylide you would have a problem. You could form the ylide from deprotonation of the desired ethyl group, or you could form an ylide by deprotonating any of the 3 methyl groups. This would give rise to a mixture of ylides and consequently a mixture of products. On the other hand, there are no protons on triphenyl phosphine that can be removed once the phosphonium salt is formed.
The following additional reasons also favor usage of triphenyl phosphine:
- it is cheap
- it is very cheap
- it is a solid and easy to handle and weigh out, many of the smaller trialkyl phosphines are liquids
- triphenyl phosphine is less toxic and doesn't smell as bad as many of the smaller trialkyl phosphines
2) Why would the lone pair on P attack the C of the alkyl halide in the
first place? Isn't the lone pair in resonance with the benzene ring?
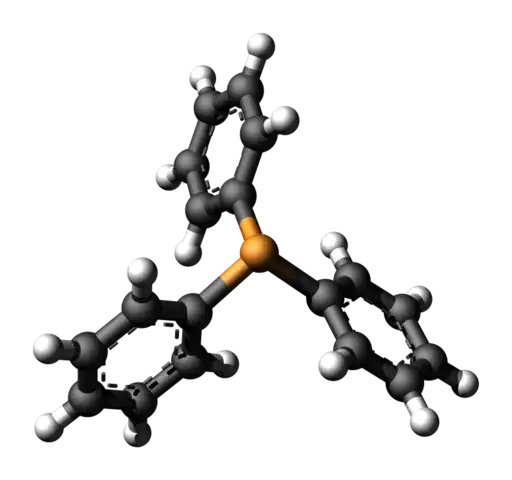
Triphenyl phosphine has the aromatic rings arranged in a non-planar, propeller-like geometry which makes delocalization less likely. Also $\ce{2p-3p}$ overlap would be involved which is not as effective as $\ce{2p-2p}$ overlap.
3) Why is butyllithium used particularly. Can't any other reagent be
used to abstract H?
In the phosphonium salt, the protons alpha to phosphorous are not very acidic (pKa ~ 20-35), a strong base is required to remove them. Other strong bases could be used, but BuLi is commercially available in high purity at a reasonable cost. When BuLi abstracts the proton butane is formed and escapes as a gas - one less side product to worry about separating out.
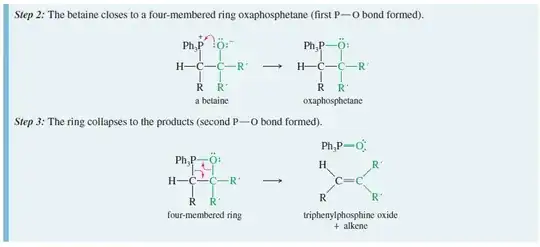
4) Why is the four membered ring formed in step 2?
The 4-membered ring may not be as strained as you imagine. Remember, phosphorous is in the third row of the Periodic Table. It has longer bond lengths and can tolerate smaller bond angles.
5) Why does the ring collapse in step 3?
The bond between phosphorous and oxygen is an extremely strong bond, in other words its formation is very exothermic. Therefore, this last step is being driven by thermodynamics. It is the exothermicity of this step that drives the reaction to completion.

2) Why would the lone pair on P attack the C of the alkyl halide in the first place? Isn't the lone pair in resonance with the benzene ring?

What if I know the answer to questions 1) and 2) from your post, but I do not know how to answer questions 3), 4), and 5)?I might not feel that I can post anything since I cannot answer all five parts. If you post them separately, then I can answer the ones I know how to answer and leave the others for people who know more than I do. – Ben Norris Jun 11 '15 at 11:54