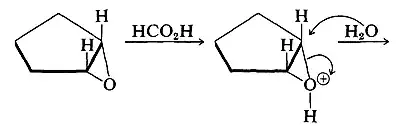
In the trans-hydroxylation of alkenes to give alcohols, there is a formation of epoxide.
Does the reaction stop here if we don't add water?
Even though if we add water, why does the product depends on which medium the reaction is carried whether acidic or basic? My text says the ring opens differently for basic and acidic medium, but both finally gives us a trans-vicinal-diol.