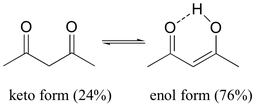
My reasoning for the somewhat implausible "$\mathrm{sp^2}$" character of the hydrogen between these two oxygens on the enol structure of the right comes from recognizing that if it were, then we would have 6 $\mathrm{sp^2}$ atoms with 6 π-electrons, so maybe that extra bit of instability from having a higher orbital on hydrogen could be sort of taken care of by being stable like an aromatic compound and forming a hydrogen bond.
So how could I go about figuring out if this is true, either with MO theory or something else?
(source: ucdavis.edu)