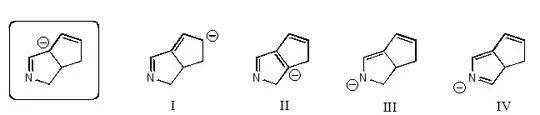
given this picture, which resonance structure is correct? I'm thinking 1 and 3. You can't have 2 because the carbon which originally had a negative charge would be making 5 bonds, and you can't have 4 because the carbon on the right side of the new double bond would also have 5 bonds.
So is this reasoning correct? I'm just having a bunch of trouble drawing resonance structures.