Find the major product of the following reaction.

Attempt:
First step would be the formation of oxonium ion. After that, there are two possibilities. Either the upper double bond or the lower bond will shift and create a carbocation (A and B)

In product A there are two possible migrations. The better migrating group is the carbon which is a part of the second ring. But it can't migrate because of formation of a bridged carbocation. So methyl group migrates.

If B carbocation is formed, then the better migrating group can attack the carbocation.
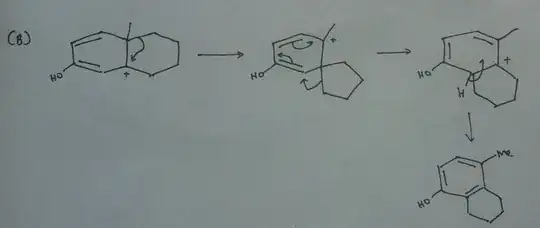
My teacher told that the product A is wrong because a weaker migrating group is acting while in B, the stronger migrating group is acting. Is this correct? Because B involves ring contraction and more steps.