The actual number of resonance structures is inconsequential: what matters is whether they are significant contributors to the resonance hybrid. This issue has been discussed at length, particularly in the case of carboxylic acids vs phenols.
Along this line of thought, the resonance structures that involve cationic carbon with six valence electrons are not really worth considering. Octet-complete resonance structures are always much more major contributors than octet-deficient ones.
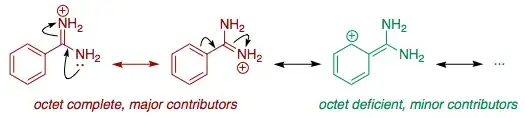
If we only count octet-complete resonance structures of the conjugate acids, then guanidine (A) wins hands down with three; the two amidines (B) and (C) both have two; and dimethylamine (D) only has one.
Between (B) and (C) the choice is tougher. You might be inclined to think that this is where the minor contributors come in: surely benzamidine (C) is more basic than acetamidine (B), since its conjugate base has some of these minor resonance contributors. However, experiment shows that the order of acidity is the other way round: benzamidine (pKa 11.6 in water at 20 °C) is actually a weaker base than acetamidine (pKa 12.52),[1] making for an overall basicity order of (A) > (B) > (C) > (D).
The reason for this is not a resonance effect, but rather an inductive effect. It is true that the benzamidinium ion enjoys marginally more stabilisation by resonance. However, in general, sp2 carbons (and aryl groups) are more electron-withdrawing than sp3 carbons (and alkyl groups). So, the positive charge on nitrogen is also destabilised by the inductively electron-withdrawing phenyl group.
A rare case of inductive effects outweighing resonance effects, but there you are.
Reference
- Albert, A.; Goldacre, R.; Phillips, J. The strength of heterocyclic bases. J. Chem. Soc. 1948, 2240–2249. DOI: 10.1039/JR9480002240.
