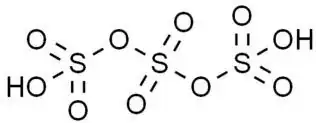
The trisulfuric acid $\ce{H2S3O10}$ hasn't been isolated yet, but it exists and has been characterized as a chain of three $[\ce{SO4}]$ tetrahedra sharing vertices and one oxygen atom from each of the terminal $[\ce{SO4}]$ tetrahedra being protonated (the structure is oversimplified and doesn't account for accurate bond order and charge distribution — for more details see e.g. Why is hydrogen sulfate put together as it is?):
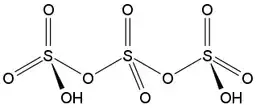
The formation of polysulfuric acids $\ce{H2S_nO_{2n+1}}$ with $n\in [1;4]$ in solutions of $\ce{SO3}$ in $\ce{H2SO4}$ has been proven by means of Raman [1] and infrared [2] spectroscopy.
According to Walrafen [1, p. 2340], $\ce{H2S3O10}$ possess at least five characteristic frequencies in Raman spectrum:
Five frequencies listed in Table III, viz., 230-250, ~325, ~350, ~685, and ~1455 cm−1, are those of $\ce{H2S3O10}.$
The bands at 230-250, ~325, and ~350 cm−1 are associated with deformation vibrations of the $\ce{S''-O-S'-O-S''}$ grouping of $\ce{H2S3O10}.$
The band at ~685 cm−1 is probably produced by $\nu_\mathrm s\text{-}\ce{S''-O-S'-O-S''},$ and the band at ~1455 is related to asymmetric valence vibrations of the central or terminal $\ce{O=S=O}$ groups.
and is mostly present in oleums with the molar fraction $x(\ce{SO3}) \approx 0.75:$
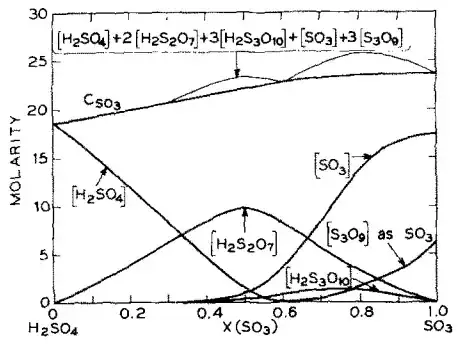
Fig. 9. Comparison of $c(\ce{SO3})$ with the sum $[\ce{H2SO4}] + 2[\ce{H2S2O7}] +3[\ce{H2S3O10}] + [\ce{SO3}] + 3[\ce{S3O9}]$ obtained from Raman data.
Stopperka [2, p. 280] assigns characteristic antisymmetric valence vibrations of central $\ce{SO2}$ group (1480 cm−1) as well as terminal $\ce{—O—SO2—OH}$ groups (1457 cm−1).
The intensities of the latter vibrations quickly decrease with an increase in molar concentration of $\ce{SO3}$:
Kleine Absorptionsmaxima bei 1457 und 1487 cm−1, die in 90proz. Oleum den antisymmetrischen $\ce{SO2}$-Valenzfrequenzen der $\ce{H2S3O10}$ zugeordnet wurden (siehe hierzu Mitt. II) gehen in ihrer Intensität ebenfalls zurück.
Bemerkenswert ist dabei, daß die schwache Bande bei 1480 cm−1 in ihrer Intensität nicht so rasch abfallt, als die bei 1467 cm−1 gelegene.
In der vorangegangenen Mitteilung (1) hatten wir die Bande bei 1480 cm−1 einer $\nu_\mathrm{as}$ $\ce{SO2}$ zugeordnet, die von einer mittelständigen $\ce{SO2}$-Gruppe der $\ce{H2S3010}$ herrührt.
Dementsprechend wurde das schwache Absorptionsmaximum bei 1457 cm−1 auf eine $\nu_\mathrm{as}$ $\ce{SO2}$ der $\ce{—O—SO2—OH}$-Endgruppe zurückgeführt.
Der mit zunehmender $\ce{SO3}$-Konzentration beobachtbare unterschiedliche Intensitätsabfall führt zu dem Schluß, daß die Konzentration an
$\ce{—O—SO2—OH}$-Endgruppen mit zunehmendem $\ce{SO3}$-Gehalt schnell absinkt, wogegen $\ce{—O—SO2}$-Gruppierungen noch deutlich bis zu relativ hohen $\ce{SO3}$-Konzentrationen nachgewiesen werden können.
A series of hydrogentrisulfate salts of alkali metals $\ce{A[HS3O10]}$ $(\ce{A} = \ce{Na}, \ce{K}, \ce{Rb}),$ (which is arguably the closest derivative of trisulfuric acid) have been isolated and characterized with single-crystal x-ray diffraction [3], also supporting the structural motif of found using spectroscopy:
![Anions linked to dimers in the crystal structure of Na[HS3O10]](../../images/bf9883b610cb88f146b11e7b7708229d.webp)
Figure 2. Anions linked to dimers in the crystal structure of $\ce{Na[HS3O10]}.$
Given $\ce{H2S3O10}$ is predicted to be Brønsted-Lewis superacid [4], its isolation is far from trivial and existence under ambient conditions is highly questionable.
References
Walrafen, G. E. Raman Spectral Studies of Oleums. J. Chem. Phys. 1964, 40 (8), 2326–2341. DOI: 10.1063/1.1725511.
Stopperka, K. Infrarotspektroskopische Untersuchungen an den flüssigen Systemen $\ce{SO3-H2O}$ und $\ce{SO3-D2O}$. III. Die Schwingungsspektren von flüssigem Schwefeltrioxid. Z. Anorg. Allg. Chem. 1966, 345 (5–6), 277–289. DOI: 10.1002/zaac.19663450506.
Schindler, L. V.; Klüner, T.; Wickleder, M. S. Towards Polysulfuric Acids: The Hydrogentrisulfate Anion $\ce{[HS3O10]}$ − in $\ce{A[HS3O10]}$ $(\ce{A} = \ce{Na}, \ce{K}, \ce{Rb}).$ Chem. Eur. J. 2016, 22 (39), 13865–13870. DOI: 10.1002/chem.201602176.
Koppel, I. A.; Burk, P.; Koppel, I.; Leito, I.; Sonoda, T.; Mishima, M. Gas-Phase Acidities of Some Neutral Brønsted Superacids: A DFT and Ab Initio Study. J. Am. Chem. Soc. 2000, 122 (21), 5114–5124. DOI: 10.1021/ja0000753.


![Anions linked to dimers in the crystal structure of Na[HS3O10]](../../images/bf9883b610cb88f146b11e7b7708229d.webp)