One synthesis of quinolones begins with the formation of an ethyl ethoxymethylenemalonate, as seen in this Organic Syntheses paper.

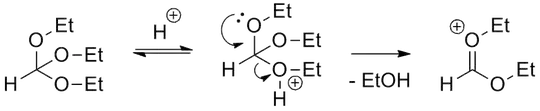
I've been asked if the malonate derivative would be formed if methylmalonate was treated with trimethyl orthoformate with a catalytic amount of $\mathrm{H^+}$, without heating. The nature of the solvent isn't mentioned, but I guess the reaction is carried out in a non-aqueous medium with an ion-exchange resin, since I think trimethyl orthoformate acts as a water trap.
My hunch is that there shouldn't be a significant quantity of product formed. We're dealing with an activated methylene, why not simply use a base and then carry on with our addition? I can't imagine how, under normal circumstances, the elimination product ethyl ethoxymethylenemalonate will be formed.
How can this reaction happen in a mechanistically reasonable way?