According to these sites:
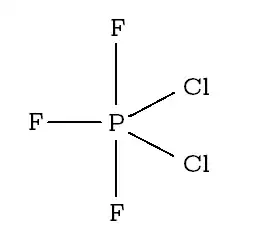
in $\ce{PCl2F3}$, both $\ce{Cl}$ and one $\ce{F}$ atom are on equatorial position, and other 2 $\ce{F}$ atoms are on axial position as follows:
The first site mentions that to minimise repulsions of $\ce{F - F}$ bonds. But this structure is in fact increasing the repulsions, and decreasing stability, since the bond angle of $\ce{F - P - F}$ bond will be about $\mathrm{90^\circ}$
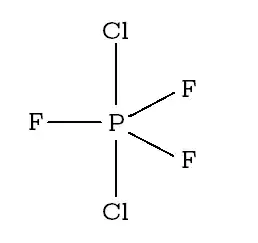
If instead all the $\ce{F}$ atoms are on equatorial position, and the 2 $\ce{Cl}$ on axial positions, then the repulsion may be further minimized.
So according to me, the structure should be:
Question: Why is my structure wrong? Please explain. Thank you!