As far as I know, there have only few truly symmetric hydrogen bonds been observed. Unquestionable is the existence of it in the bifluoride ion, $\ce{[F-H-F]-}$, see also here. There are a couple of more, like in a water hydroxyl complex $\ce{[HO\bond{~}H2O]- @Cu($110$)}$,[1] or in the hydrogen disuperoxide anion $\ce{[O2-H-O2]-}$.[2]
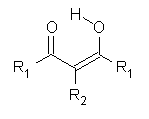
Long time it was believed that malonaldehyde and acetylacetone[3] form keto-enol tautomers with symmetric hydrogen bonds. The reason for this is the lack of resolution of the experiments.
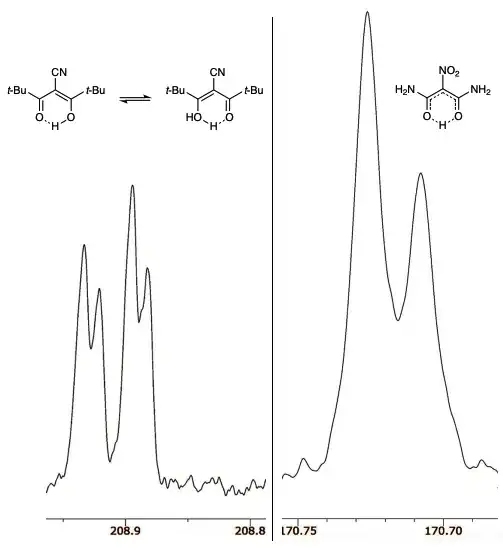
I recently came across the article about substituted malonaldehydes.[4] According to this, if you chose R1 to be an amine and R2 a nitro group, you could end up with a single well on the potential energy surface.
However, there is one response, that left me a bit puzzled. Henry Rzepa wrote:
Searches of the Cambridge crystal structure database will reveal quite a number of instances of symmetrical hydrogen bonds. These are mostly homonuclear, ie N…H…N or O…H…O. Years ago, we came across a more unusual example, of an (almost) symmetrical N….H….O heteronuclear example (http://dx.doi.org/10.1039/C39890001722 ).
So apparently there exist many. Unfortunately, he doesn't extend us the courtesy of even a single example. If you look on Google Scholar, you do indeed find a few examples of symmetric hydrogen bonds. However, most of them are either "crystallograpically" symmetric, for charged species, or both.
Also Evangelista et al. conclude their paper about malonaldehydes with the following statement, which leads me to believe, that there is no such thing as a symmetrical hydrogen bond in a neutral molecule:
A remaining goal is the identification of a substituted malonaldehyde with no barrier at all, i.e., a C2v equilibrium geometry.
Since then a few more years have passed, but my search did not turn up any more results.
Do symmetric hydrogen bonds in neutral molecules exist?
- T. Kumagai, M. Kaizu, H. Okuyama, S. Hatta, T. Aruga, I. Hamada, and Y. Morikawa, Phys. Rev. B 2010, 81, 045402
- S.J Knak Jensen , I.G Csizmadia, Chem. Phys. Lett. 2000, 319 (3-4), 220-222.
- Daryl L. Howard, Henrik G. Kjaergaard, Jing Huang, and Markus Meuwly, J. Phys. Chem. A 2015, 119 (29), 7980–7990.
- Steven Bachrach, 03 Feb 2009, Malonaldehydes: searching for short hydrogen bonds Also: Jacqueline C. Hargis, Francesco A. Evangelista, Justin B. Ingels and Henry F. Schaefer III, J. Am. Chem. Soc. 2008, 130 (51), 17471–17478.