Anywhere I look, I get 3 resonating structures for CO molecule, like in this answer.
However, according to the rules stated for drawing resonating structures in this site, I wonder why there can't be this resonating structure as well?
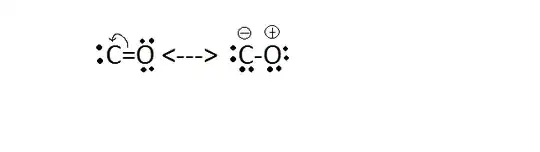 Although it will be very unstable (due to lesser number of covalent bonds, positive charge on more electronegative atom), yet a minute contribution of this structure must in present in the resonance hybrid of the CO molecule. Then why is this structure not shown anywhere?
Although it will be very unstable (due to lesser number of covalent bonds, positive charge on more electronegative atom), yet a minute contribution of this structure must in present in the resonance hybrid of the CO molecule. Then why is this structure not shown anywhere?
Asked
Active
Viewed 1.3k times
0
Debarun Mukherjee
- 273
- 2
- 3
- 8
-
4For exactly the reason you have said. It contributes a very minor component to the overall structure. There are an awful lot of possible resonance structures but very few of them are significant contributors. – bon Jun 10 '16 at 09:17
1 Answers
2
You have said it yourself. There are many possible resonance structures but most of them have a negligible contribution to the overall structure. Your proposed structure is one of them.
The major resonance contributors of carbon monoxide are: $$\ce{\!\overset{\ominus}{C}#\overset{\oplus}{O}\! ~<->~ \!C=O\! ~<->~\!\overset{\oplus}{C}-\overset{\ominus}{O}\!}$$
bon
- 15,369
- 13
- 62
- 91
-
The carbon atoms in the last two contributing canonical forms are not in octet condition. Isn't that a problem? – MrAP Aug 22 '17 at 18:49