In molecular orbital theory, please explain why cyanide ion combines with metal from the carbon end and not from the nitrogen end?
Asked
Active
Viewed 1,108 times
4
-
1It can be other way round, depending on cation. Why this theory not another? You need sth more advanced then HSAB? – Mithoron Aug 21 '16 at 20:50
-
agree with Mith, HSAB is the best explanation imo since cyanide is ambidentate, but I guess if you just want an explanation of why it can coordinate via C then I suppose the shape of the HOMO would explain it. That doesn't mean it always coordinates via C. – orthocresol Aug 22 '16 at 13:38
1 Answers
4
In this answer of Martin’s, you can find a molecular orbital diagram of $\ce{CO}$. The corresponding diagram for cyanide, $\ce{CN-}$ is essentially identical, there will only be different orbital energies and very slightly different extends of the lobes.
When forming a coordinate bond to a metal centre, cyanide will primarily attack with its highest occupied molecular orbital, the HOMO, since it is a nucleophile (attacking a positively polarised metal centre). You can also see the HOMO depicted as the bottom image of the following set as taken from Wikipedia:
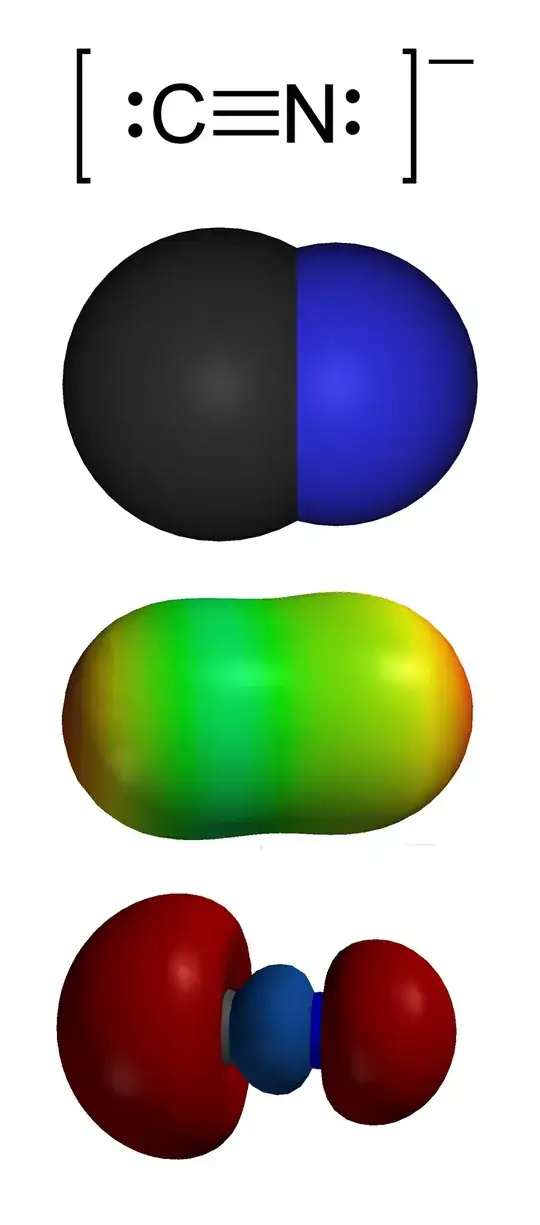
Note the large lobe extending outwards on carbon’s side. An attack with this lobe is greatly preferred, hence why isocyanides are comparatively rare.