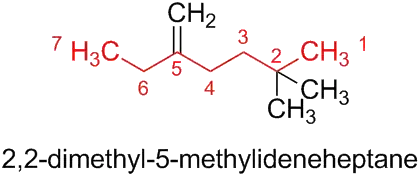
My professor posted this question as practice in naming compounds and provided the answer. I have stared at this for 30 minutes and cannot seem to figure out how he got this name out of it... Can someone please explain.
-
1Could you show us what you've tried? – schneiderfelipe Feb 02 '17 at 17:30
-
Try watching some Khan Academy videos about naming organic molecules (naming alkenes is in another section, but you could find it easily on your own). Then come back and answer your own question. – Don_S Feb 02 '17 at 17:39
-
Related: How do you name an alkene using IUPAC rules if the longest carbon chain in this alkene does not include the double bond? – Feb 02 '17 at 18:23
-
@Don_S I do not recommend to use Khan Academy for nomenclature of organic chemistry. Many names that are shown in these videos are based on obsolete rules from 1993 or even 1979, or simply wrong. They do not cite any actual IUPAC recommendations. Even in case an example name is correct, the corresponding explanations are often misleading or completely wrong. – Feb 02 '17 at 18:46
-
Thanks, @Loong, I wasn't aware of that because the videos were going along the same lines of how I studies first year organic chemistry 7 years ago. I saw your comment above and went through the related question, which made me realize it is time I update my knowledge in this field... – Don_S Feb 02 '17 at 18:54
1 Answers
The names that are proposed in the question are in accordance with obsolete IUPAC recommendations:
- 2-ethyl-5,5-dimethyl-1-hexene (1979 recommendations)
- 2-ethyl-5,5-dimethylhex-1-ene (1993 recommendations)
The corresponding rule in the 1979 recommendations is Rule A-3.4:
3.4 – Unsaturated branched acyclic hydrocarbons are named as derivatives of the unbranched hydrocarbons which contain the maximum number of double and triple bonds. If there are two or more chains competing for selection as the chain with the maximum number of unsaturated bonds, then the choice goes to (1) that one with the greatest number of carbon atoms; (2) the number of carbon atoms being equal, that one containing the maximum number of double bonds. In other respects, the same principles apply as for naming saturated branched acyclic hydrocarbons. The chain is so numbered as to give the lowest possible numbers to double and triple bonds in accordance with Rule A-3.3.
Therefore, the parent structure is the unbranched chain that contains the maximum number of double and triple bonds (here: one double bond). Since there is still a choice, the parent structure is the longest chain that contains the double bond, i.e. hexene. Numbering starts at the end of the chain that gives the lowest numbers to the double bond, i.e. 1-hexene. The 1-hexene is substituted using the usual principles of substitutive nomenclature, which yields 2-ethyl-5,5-dimethyl-1-hexene.
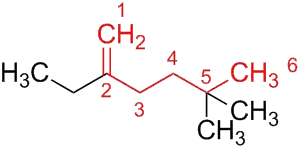
According to the 1993 recommendations, locants are placed immediately before the part of the name to which they relate. Therefore, the name 2-ethyl-5,5-dimethyl-1-hexene is changed to 2-ethyl-5,5-dimethylhex-1-ene.
The current version of Nomenclature of Organic Chemistry – IUPAC Recommendations and Preferred Names 2013 (Blue Book) incorporates a major change from earlier recommendations given in 1979 and 1993.
In acyclic parent structures the order of seniority between unsaturation and length of chain given in earlier recommendations is reversed. Thus, the first criterion to be considered in choosing a preferred parent acyclic chain is the length of the chain; unsaturation is now the second.
Therefore, the parent structure of the compound that is given in the question is now the longest chain, i.e. heptane. The heptane is substituted using the usual principles of substitutive nomenclature; i.e. the prefixes (here: ‘methyl’ and ‘methylidene’) are considered together in a series of increasing numerical order. This yields the preferred IUPAC name 2,2-dimethyl-5-methylideneheptane rather than 6,6-dimethyl-3-methyleneheptane since the locant set ‘2,2,5’ is lower than ‘3,6,6’.