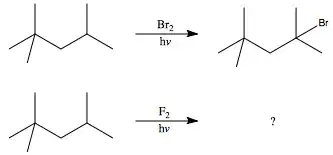
I understand what structure is formed by using $\ce{Br2}$ to brominate this alkane, and I know that $\ce{F2}$ will be less selective in its abstraction of hydrogen. However, how can I know where $\ce{F2}$ will primarily react with this alkane? I just know that $\ce{F2}$ will react more readily and be less selective.
-
4"Less selective" is a bit of an understatement. F2 will react everywhere. – Ivan Neretin Feb 14 '17 at 05:27
1 Answers
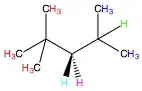
There is nearly no thermodynamic aspect to this reaction. The activation energy for any alkane hydrogen abstraction by a fluorine radical is essentially 0$^{[1]}$. This means that there is an equally likely chance of fluorinating at any given position. In this case, we need only know the proportions of homotopic hydrogen. Homotopic hydrogen are labelled with the same colors in the molecule:
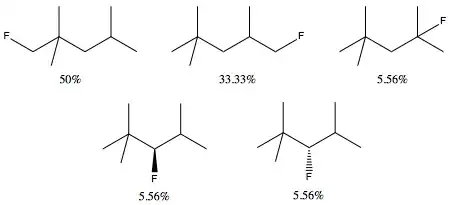
The proportion in decreasing order is 9:6:1:1:1. That means that we should expect about the following yield of monofluorinated products:
As an aside, you would never realistically expect to obtain a monofluorinated product. The fluorination of alkanes is violent, extremely fast, and only gets faster upon fluorination.
$^{[1]}$ Chemistry StackExchange, Why are free radicals so reactive?