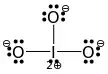
After doing the Lewis structure of Bromate Ion and in order to find the molecular geometry using VESPR method, we have:
central atom: Br
Electrons of the central atom: 7
Electrons that contribute the 3 Oxygens : 3
Electrons that contribute the central atom for the π bonds: -2
Charge of ion (negative) : 1
Total electrons : 9
But if we divide 9 with 2 in order to find the σ-bonding pairs, we get 4,5. what is wrong? Because after searching the molecular geometry of BrO3- it says that it is trigonal pyramid.