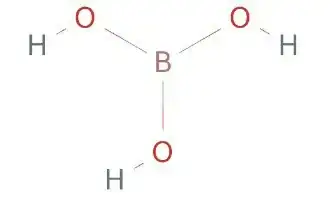
What should be the hybridization of the oxygen atom?
- A) It should be $\mathrm{sp^2}$ as 1 lone pair must be in the unhybridized orbital for back bonding with boron which has a vacant p-orbital
- B)It should be $\mathrm{sp^3}$ (2bp + 2lp)
(In my book it is given as $\mathrm{sp^3}$ but I cannot find a reason to rule out option A. I am not convinced with option B.)