This excellent answer describes the appearance of solvated electrons in ammonia as first a blue, and then metallic color. It is produced by dissolving sodium in ammonia at low temperature. According to Wikipedia this is called an electride:
An electride is an ionic compound in which an electron is the anion. Solutions of alkali metals in ammonia are electride salts. In the case of sodium, these blue solutions consist of $\ce{[Na(NH3)6]+}$ and solvated electrons:
$$\ce{Na + 6 NH3 → [Na(NH3)6]+e−}$$
The cation $\ce{[Na(NH3)6]+}$ is an octahedral coordination complex.
Question: How to interpret the shape in this representation of an electride?
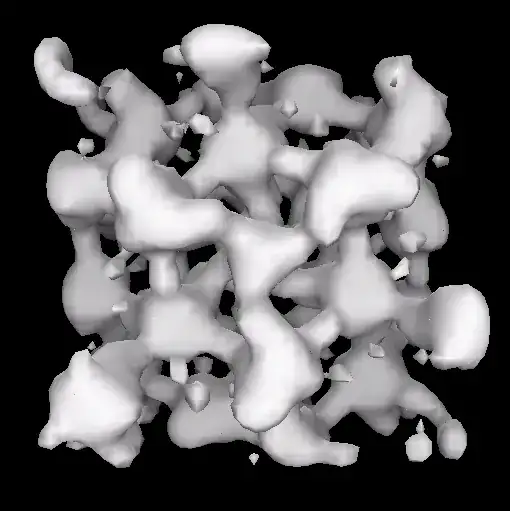
Included in the Wikipedia article is the image shown below. Is it fairly self-evident to chemists what this image represents? If so could someone help me understand it? I see little things which might represent the location of solvated electrons, and a larger continuous/contiguous? surface which might represent all of the ions and molecules and the fact that any moment most of them are in some degree of bonding with each other, but that's just my guess. What defines the surface, some sort of threshold on electron density?
In this case the original source might be hard to track down, so an "educated guess" by someone familliar with this kind of representation would be welcome.
I'd like to read more about this kind of 3D representation of an ionic solution, so an introductory source would be appreciated.
below: "Cavities and channels in an electride", from here.