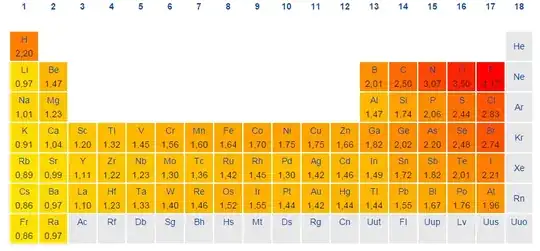
I know that an atom that an atom is more electronegative as it moves up a group or right on a period (in terms of the periodic table).
So if you have two atom that is diagonally across from each other, which one is more electronegative? Do you prioritize groups or periods when determining electronegativity?
For example: Which is more electronegative - Sulfur, or Bromine?
Finally, does this priority also work for first ionization and atomic radius trends. (in other words, if you prioritize groups when determining electronegativity, do you also prioritize groups when determining the other two periodic table trends?)
My guess is that they would be equal, but I may be wrong.
P.S. I would be most likely to give best answer to the person who answers all my questions as a numbered list.