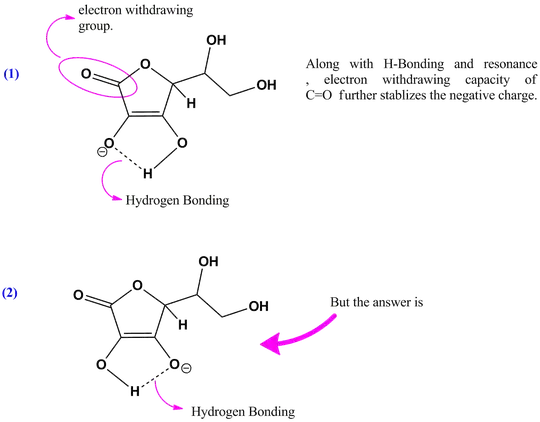
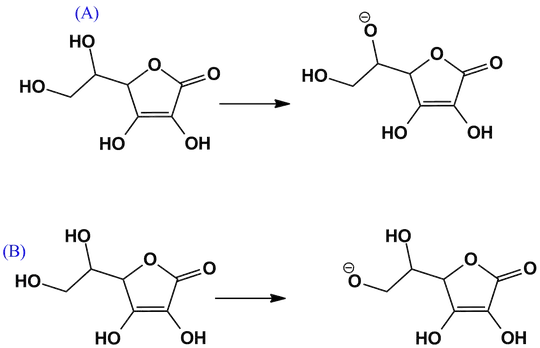
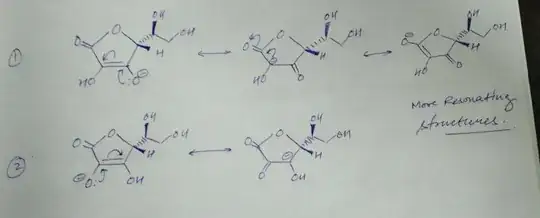
I thought it would be the lower first $\ce{-OH}$ because it's close to the double bond with oxygen, so I thought there would be more electron withdrawal from the oxygen atom due to its electronegativity.But the correct answer is actually the second lower $\ce{-OH}$, which I really don't understand
My attempt to the question: