I've seen the related questions 1 and 2, they're not duplicates
So. when introducing Red P+HI reduction, our teacher said that it is the strongest reducing agent, and it can reduce any functional group i.e. all of these: carboxylic acid, acid anhydride, ether, amide, acid halide, carbonyl, and nitrile
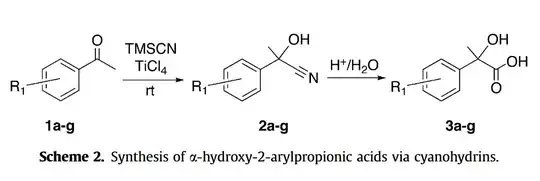
However, today I stumbled upon a question in my book involving a step in the synthesis of ibuprofen, where they did not reduce the carboxylic acid group even after using RedP+HI. I searched a lot on the internet, and finally came across this paper which confirms this:
paper - notice they've only reduced the alcohol in step (ii)
This now makes me wonder if what I was taught is actually correct. Which brings me to the question:
Does RedP+HI really reduce all carbon functional groups to alkane?