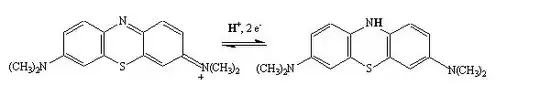
Why does methylene blue (which is initially blue) reduce to form a colorless solution?
I was told by my teacher that it has to do with the presence of an additional double bond on methylene blue, but I can't figure out or find any source on the internet that claims so.