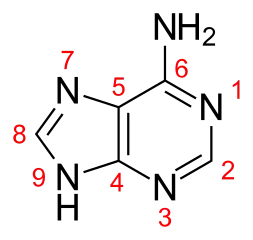
According to Ref.1:
Adenine undergoes two ionization reactions. Density function calculations in the gas phase carried out by Russo et al. (1998) indicate that protonation occurs preferentially on the N1 nitrogen of the 6-member ring, in good agreement with the assignment given by Christensen et al. (1970b). The anionic species with a proton-deficient 5-member ring is the most stable.
Here, "Russo (1998)" is the Ref.2, which says (emphasis added):
HOMO of adenine is formed essentially by the lone pairs of all nitrogens. The orbital of the amine group contributes with the higher coefficient, and that of $\ce{N}$1 with the lower one. On the basis of this information, $\ce{N}$1 protonation might be the less favorable process. On the contrary, the molecular electrostatic potential map (Fig. 5) indicates that $\ce{N}$1, $\ce{N}$3, and $\ce{N}$7 are the better candidates for proton attachment. In particular, the $\ce{N}$1 and $\ce{N}$3 regions appear to be favored over $\ce{N}$7. Experimentally, the preferred protonation sites of adenine are $\ce{N}$1 and $\ce{N}$3 with the former slightly favored.[reference 18] Our computation favors protonation at $\ce{N}$1 over those at $\ce{N}$3 and $\ce{N}$7 by $1.12$ and $\pu{7.38 kcal mol-1}$, respectively. This trend is in agreement with previous theoretical computations.[reference 17] From bond orders analysis (see Fig. 6), and due to the small energy difference it is possible to view the $\ce{N}$1 and $\ce{N}$3 protonated forms in $\ce{N}$1 the double bonds occur in $\ce{C}$4—$\ce{C}$5 and in the $\ce{C}$2—$\ce{N}$3 positions, whereas, in the second, in the $\ce{C}$4—$\ce{C}$5 and $\ce{N}$1—$\ce{C}$2 positions as two resonance hybrids of a unique structure with a consistent charge delocalization. This observation allows better insight into the reason for which the protonation on the five-membered ring is unfavorable. In fact, as shown in Figure 6, the positive charge appears to be distributed only on the $\ce{C}$8 and $\ce{N}$9 atoms, because it is evident that no interactions exist between the two rings upon protonation.
Note: "Christensen" of the Ref.1 is the same as reference 18 of the Ref.2, and is listed here as Ref.3. Reference 18 mentioned in the Ref.2 is listed here as Ref.4.
References:
- Erik Balodis, Melerin Madekufamba, Liliana N. Trevani, and Peter R. Tremaine, "Ionization constants and thermal stabilities of uracil and adenine under hydrothermal conditions as measured by in situ UV–visible spectroscopy," Geochimica et Cosmochimica Acta 2012, 93, 182-204 (DOI: https://doi.org/10.1016/j.gca.2012.06.023).
- Nino Russo, Marirosa Toscano, André Grand, and Franck Jolibois, "Protonation of thymine, cytosine, adenine, and guanine DNA nucleic acid bases: Theoretical investigation into the framework of density functional theory," Computational Chemistry 1998, 19(9), 989-1000 (DOI: https://doi.org/10.1002/(SICI)1096-987X(19980715)19:9<989::AID-JCC1>3.0.CO;2-F).
- James J. Christensen, J. Howard Rytting, and Reed M. Izatt, "Thermodynamic pK, ΔH°, and ΔS° and ΔCp° values for proton dissociation from several purines and their nucleosides in aqueous solution," Biochemistry 1970, 9(25), 4907–4913 (DOI: https://doi.org/10.1021/bi00827a012).
- Janet E. Del Bene, "Molecular orbital study of the protonation of DNA bases," J. Phys. Chem. 1983, 87(2), 367–371 (DOI: https://doi.org/10.1021/j100225a040).