See following image: 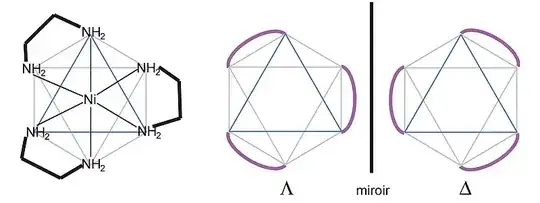
Look at the purple loop (en) on upper left hand side structure. Now, if you rotate the whole molecule ${90^o}$ clockwise around an axis going through top $\ce {NH2}$- $\ce {Ni}$-bottom $\ce {NH2}$, you will get that loop overlapping on similar loop on the mirror image. But, now look what happens to the other two loops? They are not overlapping on other two loops on mirror image. For example, the purple loop on bottom of left hand side structure will move to back side of the same side (it would be ${180^o}$ far from similar loop in the mirror image). Thus this molecule would not superimpose with its mirror image.
 Isn't this molecule achiral? I mean if I flip the mirror image on right by 180 degrees(like we do to determine chirality in tetrahedral carbons) it would be superimposeable? But apparently it is chiral. How am I flipping wrong?
Isn't this molecule achiral? I mean if I flip the mirror image on right by 180 degrees(like we do to determine chirality in tetrahedral carbons) it would be superimposeable? But apparently it is chiral. How am I flipping wrong?
