Question:
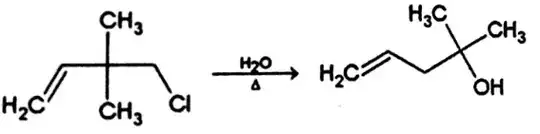
Predict the mechanism for the following reaction:
Attempt:
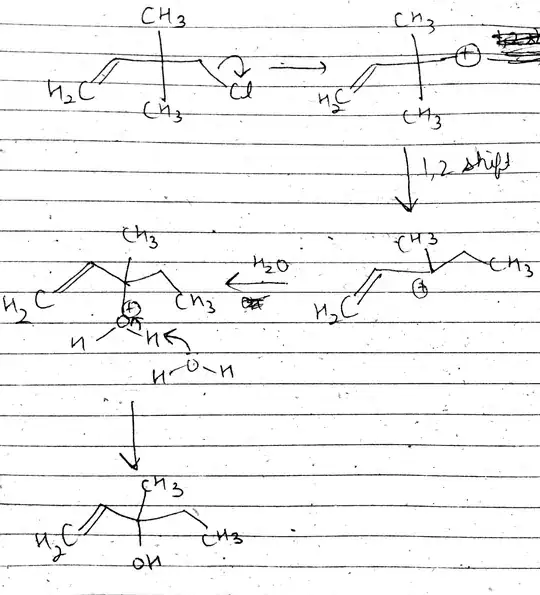
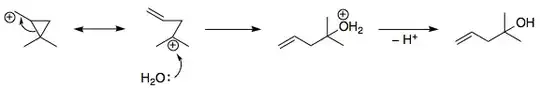
I thought it will proceed through SN1 mechanism as $\ce{H_2O}$ is involved (solvolysis). So I proceeded with the formation of a primary carbocation, which then rearranges via 1,2 methyl shift. Then the nucleophilic attack of $\ce{OH^{-}}$ leads to the following product, which is different form the required one.
Can any further steps lead to the required product or am I wrong from the start (most probably)?